The secretory pathway:
a key control point in protein biology
The secretory pathway is a fundamental process in human biology. It enables cells to manufacture, process, and release proteins that act beyond their own boundaries, such as cytokines or surface receptors that orchestrate immune responses. Proteins destined for secretion or membrane insertion carry a signal peptide or transmembrane domain, which ensures their recognition by the Sec61 translocon.
Because many disease-driving proteins rely on secretion, the Sec61 translocon represents a highly potent control point. At the Sec61 translocon, signal peptide recognition triggers the translocation of nascent proteins into the endoplasmic reticulum, where they undergo folding, maturation, and trafficking to their final destination.
Powered by machine-learning
A large, well-characterized chemical space spanning multiple Sec61 translocon inhibitor families, combined with extensive biological datasets, including proteomics and tailored cell line libraries, enables the application of machine-learning–guided, rational small-molecule drug design. This approach allows precise targeting of disease-driving proteins while preserving normal cellular function.
By intervening in the protein secretion pathway at the point of synthesis, Enodia is stopping a faulty product at the factory line rather than recalling it after distribution. This strategy enables the degradation of disease-driving proteins before they mature or are released, creating opportunities across multiple therapeutic areas, including inflammatory and autoimmune diseases.
Targeting the Sec61 translocon: Intercepting disease-driving proteins early
By targeting the point of synthesis, disease-driving proteins can be intercepted at the very start of their life cycle. Instead of being released to fuel disease, they are redirected to intracellular degradation through our innovative approach.
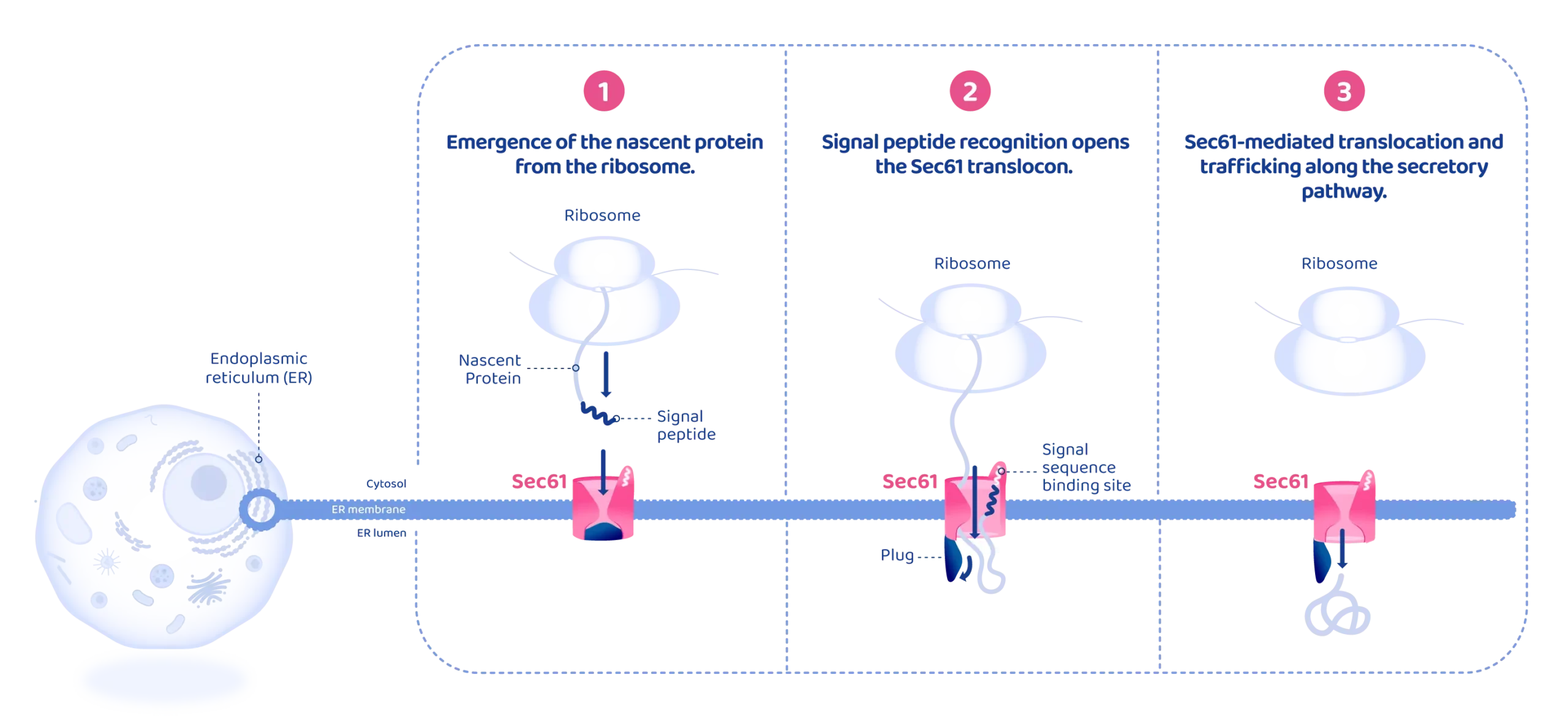
- Nascent secretory proteins are targeted to the ER as they emerge from the ribosome.
As the protein is synthesized by the ribosome, an N-terminal signal peptide or transmembrane segment emerges from the ribosomal exit tunnel, marking the protein for ER targeting.
The ribosome–nascent chain complex is then delivered to the ER membrane, where the ribosome docks onto the Sec61 translocon, driving the growing polypeptide into the channel. - Signal peptide recognition opens the Sec61 translocon.
The signal peptide inserts into the lateral gate of Sec61 and binds to the signal peptide–binding site. This interaction stabilizes the nascent chain and initiates structural rearrangements of the translocon.
Signal peptide insertion induces conformational changes that open the Sec61 channel both: laterally, toward the lipid bilayer, and vertically, through displacement of the luminal plug.
This converts Sec61 from a closed to an open, translocation-competent state.
The growing polypeptide passes through the open channel in a looped configuration. Hydrophilic regions move through the central pore toward the ER lumen, while hydrophobic segments interact with the lateral gate. - Sec61-mediated translocation and trafficking along the secretory pathway.
• Transmembrane segments are laterally released from Sec61 into the lipid bilayer.
• Soluble proteins fully translocate into the ER lumen.
Once the signal peptide is cleaved, the protein folds and matures within the ER before being targeted to the plasma membrane or secreted via the secretory pathway.